A narrative review of ADME testing platforms
Kratek pregled platform za ADME testiranje
DOI:
https://doi.org/10.18690/actabiomed.275Keywords:
pharmacokinetics, ADME, in vitro models, drug interaction studies, multi-organ modelsAbstract
Background: Significant progress has been made in absorption, distribution, metabolism, excretion (ADME) models, but the creation of platforms that reduce animal testing and research costs is still challenging. The increasing complexity of pharmacokinetic interactions underscores the need for reliable, reproducible ADME models, which are essential for drug development and safety to prevent serious clinical complications and hospitalizations.
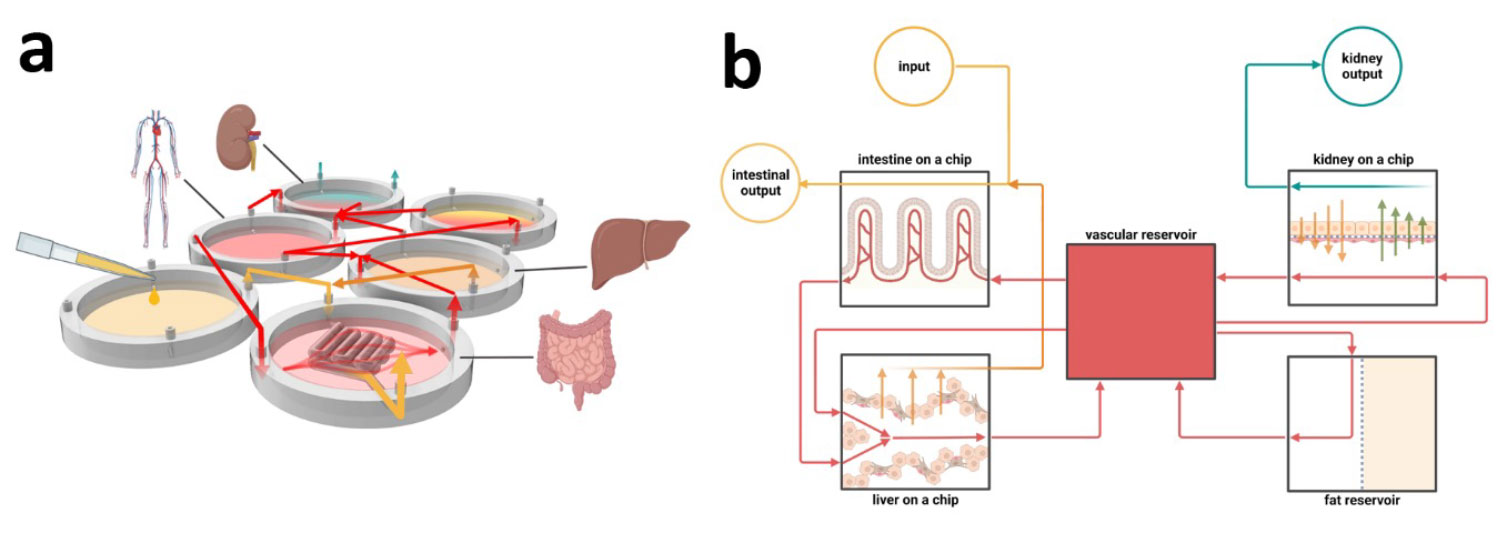
Aims: This narrative review aimed to discuss multi-organ, function-based ADME platforms and evaluate recent developments in microphysiological systems (MPS) emphasizing functional accuracy.
Methods: We evaluated MPS models developed to improve the relevance of in vitro drug interaction studies by emphasizing critical tissue function over detailed anatomical mimicry. We also highlighted the strengths and limitations of these platforms in replicating the full complexity and functionality of native tissues.
Results: Despite making progress in structural replication, MPS models often lack the functional fidelity required for accurate drug testing. Models that emphasize functionality rather than complex structural details show promise in producing results relevant to humans, thus providing an alternative to animal testing.
Conclusions: A shift in focus from anatomical complexity to mimicking key tissue functions is required to fully realize the potential of MPS in ADME studies. Addressing the current limitations of biomedical engineering in this way may support the ethical, scientific, and regulatory goals of preclinical research. MPS can bridge the gap between traditional models and human-relevant systems by emphasizing functional accuracy, thus increasing the translational value of ADME studies and contributing to effective, ethical drug discovery.
Downloads
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Tina Maver, Boštjan Vihar, Uroš Maver (Author)

This work is licensed under a Creative Commons Attribution 4.0 International License.