Endocrine activity assessment approaches: From mechanistic bioassays to adverse pathway outcomes
Pristopi za ocenjevanje endokrine aktivnosti: od mehanističnih bioloških testov do poti neželenih izidov
DOI:
https://doi.org/10.18690/actabiomed.292Keywords:
Endocrine-disrupting compounds, High-throughput screening, Adverse outcome pathways, Environmental risk assessmentAbstract
Purpose: To provide an integrated overview of current approaches for detecting and characterizing endocrine activity in chemicals by emphasizing the relationship between mechanistic in vitro assays, in vivo models, and regulatory frameworks.
Methods: Recent developments in high-throughput screening (HTS) platforms, validated Organisation for Economic Co-operation and Development (OECD) test guidelines, and effect-based bioassays were critically compared. The review covers representative assays for estrogenic, androgenic, thyroid, and steroidogenic pathways and evaluates the complementarity within adverse outcome pathway (AOP) frameworks. Attention was given to data integration, bioavailability considerations, and regulatory adoption under the European Union Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) and the US Environmental Protection Agency (EPA) Endocrine Disruptor Screening Program.
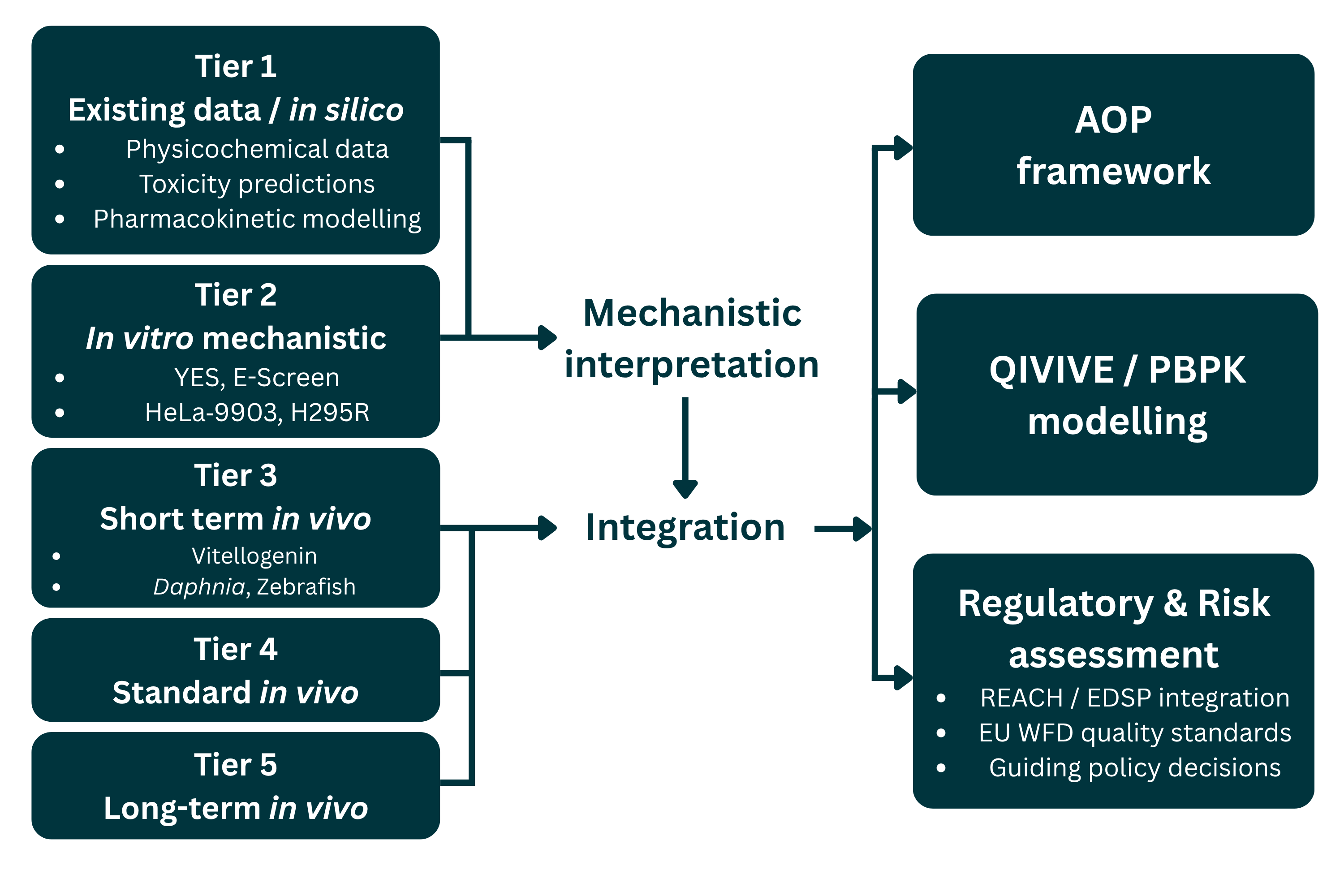
Results: Mechanistic assays, such as YES, E-Screen, HeLa-9903, and H295R, provide rapid, sensitive, and cost-effective screening for receptor-mediated and steroidogenic effects. In contrast, in vivo systems, including vitellogenin induction, Daphnia reproduction, amphibian metamorphosis, and zebrafish assays, yield organism-level confirmation. Integrating both tiers within AOP and quantitative in vitro-to-in vivo extrapolation (QIVIVE) frameworks increases predictive reliability. Despite advances in standardization, challenges remain concerning metabolic competence, inter-laboratory variability, and harmonized effect thresholds.
Conclusion: Endocrine testing science is transitioning toward mechanistically anchored, animal-reduced strategies that combine HTS, computational modeling, and effect-based monitoring. Harmonized validation criteria and performance standards will further enhance regulatory acceptance and facilitate the efficient identification of substances with endocrine activity across both human health and environmental contexts.
Downloads
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Andrej Grobin (Author)

This work is licensed under a Creative Commons Attribution 4.0 International License.